General microbial energy metabolism
Bacteria gain energy by the
transfer of electrons and protons from a reduced substrate at a lower potential
to an electron acceptor at a higher potential. The energy gained can be
calculated as:
ΔG = - n x F x Eemf
with n the number of electrons
exchanged, F Faraday’s constant (96485 C/mol) and Eemf the
thermodynamic equilibrium cell potential (also referred to as ΔV). Bacteria can perform this electron transfer on several ways, but
overall, two main classes of microbial energy metabolism are known: respiration
and fermentation. Which type of energy metabolism is used, depends on the
properties of the bacteria and the available electron donor and acceptor.
1) Respiration
During respiration, substrate is oxidized with subsequent liberation of protons
and electrons. These electrons are generally deposited on a NAD+ molecule,
which is reduced to NADH. This NADH is re-oxidized at NADH dehydrogenase, and
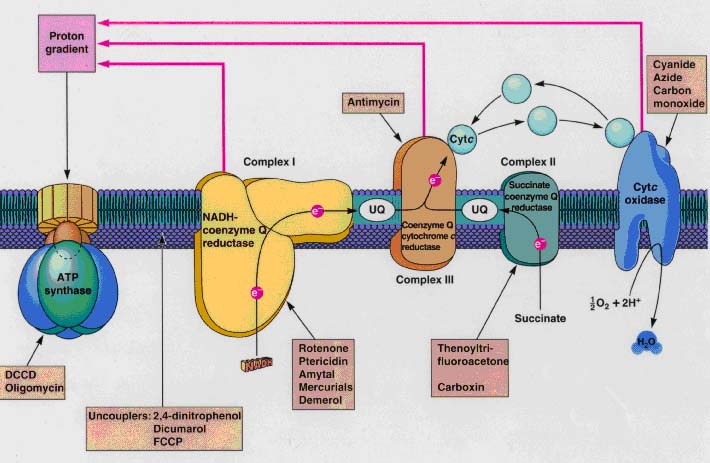
the electrons subsequently follow the electron transport chain (Figure 1.3).
Electrons and concomitantly protons can be transported through the NADH
dehydrogenase, ubiquinone, coenzyme Q or cytochrome. Every subsequent component
in this chain has a higher potential than the electron donating component. The
energy released during the electron transport enables bacteria to pump protons
outwardly to the periplasm. Thus a proton motive force is generated, enabling
activity of ATP synthase and hence the formation of ATP. All electrons not
captured for growth within the bacteria can theoretically be transported to the
electron acceptor, with concomitant conversion of carbonaceous substrate to
CO2.
2) Fermentation
Fermentative metabolic pathways are used when no readily available electron
acceptors are present in the bacterial environment. During fermentation,
bacteria will deposit part of the liberated electrons on the oxidized substrate
and hence form reduced metabolites such as ethanol and acetate but also
hydrogen and methane.
Microbial energy metabolism in MFCs
The use of respiration pathway in
MFCs was investigated by (Kim et al. 2004). They observed that the generation
of electrical current from a MFC was inhibited by various inhibitors of the
respiratory chain. The electron transport system in their MFC utilized NADH
dehydrogenase, Fe/S proteins and quinones as electron carriers, but does not
use site 2 of the electron transport chain or the terminal oxidase. Processes using
oxidative phosphorylation have regularly been observed in MFC, yielding high
energy efficiencies (Rabaey et al. 2003). Examples are consortia containing
Pseudomonas aeruginosa, Enterococcus faecium (Rabaey et al. 2004) and
Rhodoferax ferrireducens (Chaudhuri & Lovley 2003).
During fermentation the reducing
equivalents (e.g. NADH2, ferredoxin,…) originating from the
oxidation of pyruvate to acetyl-CoA need to be re-oxidised to become available
again for the bacteria. This can be done by the production of fermentation
products like lactate, acetate, butyrate, ethanol,… but hydrogen can also be
produced. However, the liberation of hydrogen from these reducing equivalents
becomes only thermodynamic feasible if the partial pressure of hydrogen is
lower than 60 Pa (Angenent et al., 2004). Furthermore, approximately 2/3 of the
electrons remain in the produced fermentation products such as acetate, while
maximum 1/3 can theoretically be used to generate current (Logan, 2004). The
1/3 electrons are possibly available for electricity generation since the
hydrogenases, that generally use the electrons to produce hydrogen gas, are
often situated at places accessible from outside by mobile electron shuttles in
the periplasm (McKinlay & Zeikus 2004).In microbial fuel cells, methane production has also
repeatedly been observed (Kim et al.) indicating that the bacteria do not fully
use the anode. The fermentation products are further oxidized at low anode
potential by anaerobic bacteria such as Geobacter species, capable to withdraw
electrons from acetate in MFC conditions (Bond & Lovley 2003).
Does the anode potential affects the
microbial energy potential?
Yes it does!.
If bacteria derive reducing
equivalents from glucose in the form of NADH, and subsequently shuttle
electrons from NADH to oxygen (not taking into account potential
decreases between NADH and the final bacterial electron shuttle), the potential
difference is:
ΔE = (+0.840V)-(-0.320V) = 1.16 V,
and the energy
to be gained (2 electrons per molecule of NADH)
ΔG = -223 kJ/mol.
If the electron acceptor is sulphate,
the potential difference decreases to approximately
ΔE = (- ±0.220V)-(-0.320V) = ± 0.10 V,
yielding a ΔG of only -19 kJ/mol. The amount of energy available for the
bacteria to grow is very low in that case.
In the anode compartment
of an MFC no oxygen is present, except the oxygen diffusing through the
membrane adjacent to the anode. If an anode is available with a higher
potential than for example sulphate present in the feed stream, the energetic
gain will be much higher for bacteria that can deliver to the anode. Hence, the
anode will become the preferred electron acceptor. If however the anode
potential is to low, electricity production will cease and fermentation
processes will start.
How does the electron transfer occur?
To obtain microbial electricity
production, the electrons released out of the substrate still need
extracellular electron transport towards the anode. Three major pathways can be
discerned:
- Membrane associated electron transfer (Bond & Lovley 2003; Kostka et al.
2002)
-
- Nanowires or electrically conductive bacterial
appendages (Reguera et al., 2005) The three pathways may interlink,
rendering the distinction less strict.
Microbial
ecology of MFCs
MFCs operated using mixed
cultures currently achieve substantially greater power densities than those
with pure cultures. In one recent test, however, an MFC showed high power
generation using a pure culture, but the same device was not tested using
acclimated mixed cultures and the cells were grown externally to the device.
Community analysis of the microorganisms that exist in MFCs has so far revealed
a great diversity in composition. We believe, based on existing
data, and new data from our individual laboratories, that many new types of
bacteria will be discovered that are capable of anodophilic electron transfer
(electron transfer to an anode) or even interspecies electron transfer
(electrons transferred between bacteria in any form).